Our Research
Driven by Discovery - Backed by Data
At Canhitt, science isn’t just what we do—it’s who we are. Our research fuels everything from platform innovation to real-world impact, and we’re proud to share the data, insights, and peer-reviewed publications that power our progress. Dive into our figures, explore our findings, and see how our cutting-edge work is shaping the future of preclinical drug discovery. This is where bold ideas meet rigorous science—and where breakthroughs begin.
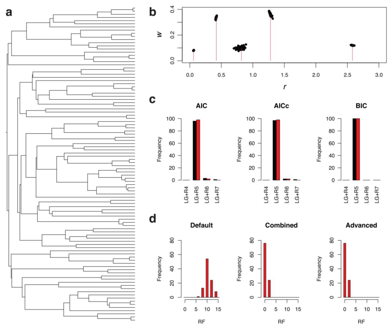
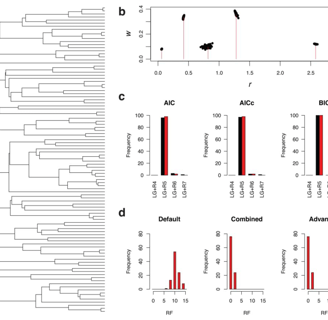
Kalyaanamoorthy, S., Minh, B., Wong, T. et al. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods 14, 587–589 (2017).
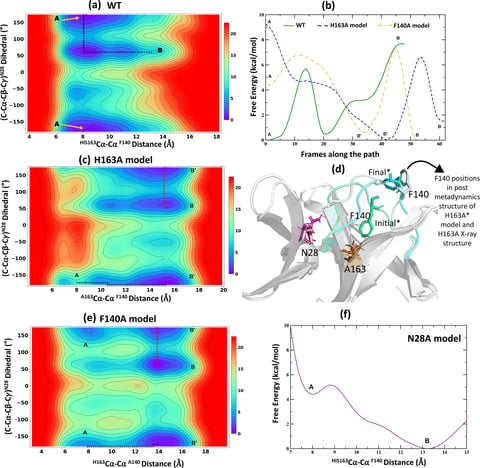
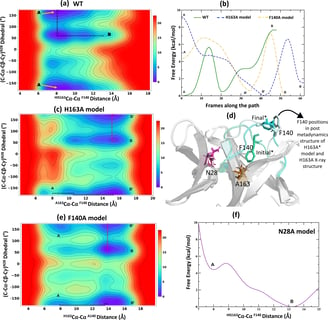
Tran, N., Dasari, S., Barwell, S.A.E. et al., The H163A mutation unravels an oxidized conformation of the SARS-CoV-2 main protease. Nat Commun 14, 5625 (2023).
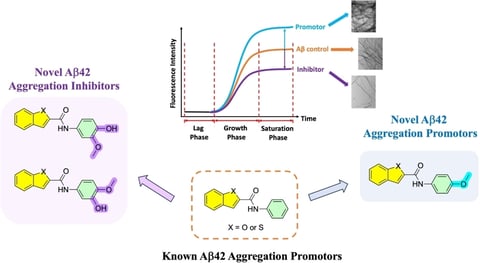
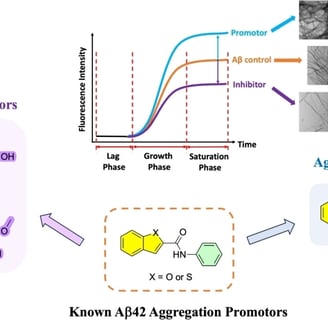
Y. Zhao, K. Singh, R. Chowdary Karuturi, A. A. Hefny, A. Shakeri, M. A. Beazely, P. P. N. Rao, ChemMedChem2024, 19, e202400198.
https://doi.org/10.1002/cmdc.202400198
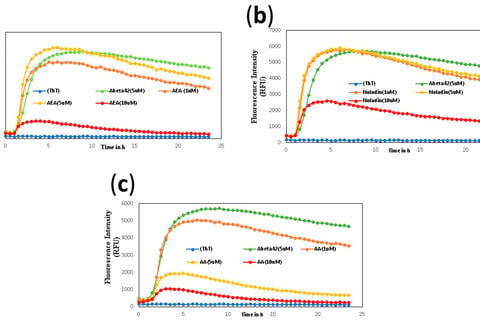
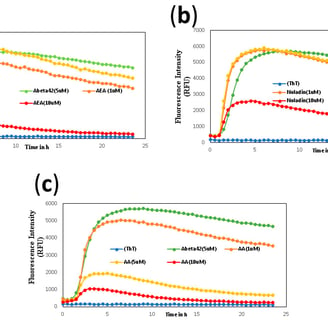
Khavandi, M., Rao, P. P. N., Beazely, M.A. Int. J. Mol. Sci. 2023, 24(2), 911
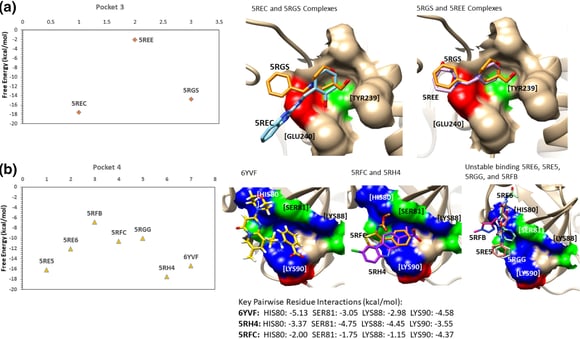
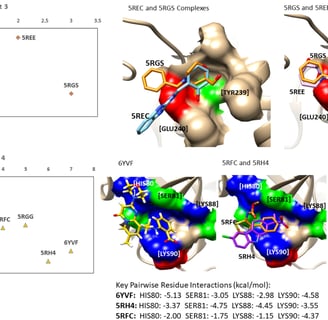
Weng, Y.L., Naik, S.R., Dingelstad, N. et al. Molecular dynamics and in silico mutagenesis on the reversible inhibitor-bound SARS-CoV-2 main protease complexes reveal the role of lateral pocket in enhancing the ligand affinity. Sci Rep 11, 7429 (2021).
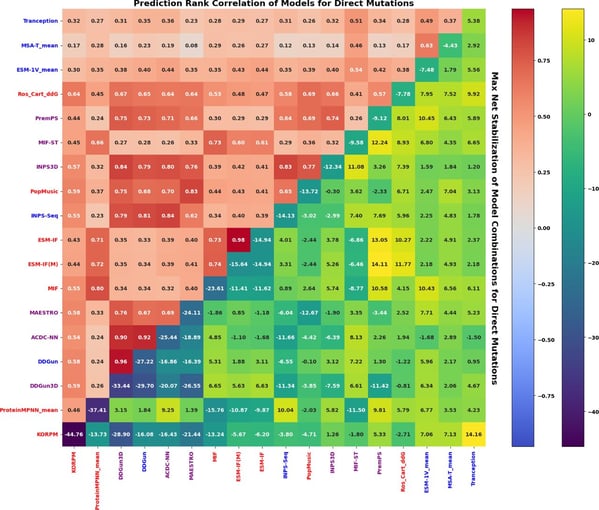
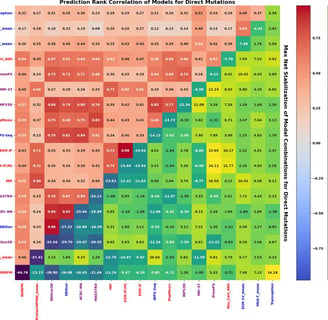
Reeves, S., Kalyaanamoorthy, S. Zero-shot transfer of protein sequence likelihood models to thermostability prediction. Nat Mach Intell 6, 1063–1076 (2024).
Catalyzing New Health Innovation
Advancing drug discovery and health technologies globally.
Research
Consultancy
contact@canhittherapeutics.com
hello@canhitt.com
© 2025. All rights reserved.
